According to the American Society of Quality:
A quality management system (QMS) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. A QMS helps coordinate and direct an organization’s activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous basis.
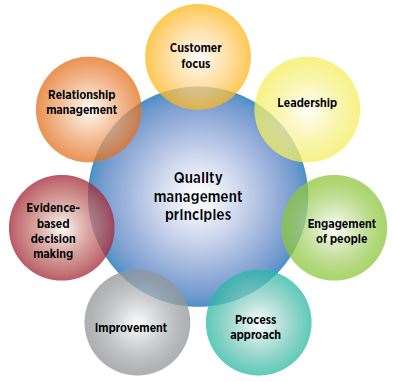
Element of a quality management system:
Quality management systems should address an organization’s unique needs; however, the elements all systems have in common include:
- The organization’s quality policy and quality objectives
- Quality manual
- Procedures, instructions, and records
- Data management
- Internal processes
- Customer satisfaction from product quality
- Improvement opportunities
- Quality analysis
The laboratory is committed to achieve Quality Standards of CBAHI, JCI and CAP. Achieve accreditation is an essential part of the strategic objectives of the laboratory.